Setting and maintaining specifications - Evaluation and manufacture of blood components - Chapter 6
The wide variability of the source material from which blood components are prepared makes it difficult to set stringent limits. Nevertheless, realistic minimum specifications should be set and complied with.
Concessionary release limits are also set for certain components that are subject to non-destructive quality monitoring, such that components that are excessively out of specification are only used therapeutically under specific circumstances and subject to formal clinical approval (Table 6.1).
All blood components tested and found outside concessionary release limits should have the testing repeated to confirm the original result and the production process should be reviewed as necessary.
Some abnormal results could have implications for the health of the donor and should be reviewed by a Designated Clinical Support Officer. Further samples or referral to an appropriate clinical service may be required for the donors of:
- Red Cells in Additive Solution, Leucocyte Depleted, where the Hct is >0.70
- Platelet components collected by apheresis, where the platelet count is below any lower concessionary release limit
- For Fresh Frozen Plasma, Leucocyte Depleted where the FVIII is <0.3IU/mL
| Blood component | Parameter | LOWER Limit (less than) | UPPER Limit (more than) |
|---|---|---|---|
| Red Cells in Additive Solution, Leucocyte Depleted | No data | No data | No data |
Component and process quality monitoring results should be subjected to statistical analysis so that trends can be identified. Guidance on appropriate approaches to statistical process monitoring is given in the Council of Europe guide,1 and in Beckman et al. (2009).2 A flowchart adapted from Beckman et al., to aid in selection of appropriate methods, is reproduced as Figure 6.1.
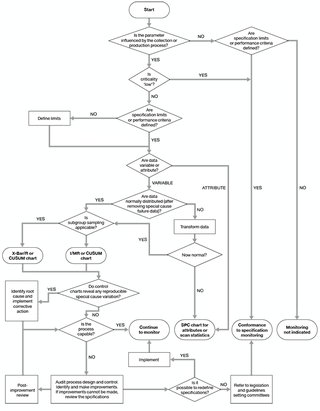
Figure 6.1 Algorithm for the selection of quality monitoring methods
If the results of analyses show a consistent trend towards the minimum requirements specified in Chapter 7, the cause should be investigated. The criteria to be investigated must be detailed in the relevant SOP together with the corrective action to be taken. The steps to be considered should include the following:
- An investigation of the collection, testing, production and distribution procedures as appropriate.
- Checking that procedures are up to date and are not being deviated from.
- Checking the operation of equipment and storage conditions (this may include reviewing validation documentation and/or revalidation).
The person responsible for quality assurance and/or production may initiate investigations beyond the scope of written procedures.